atrify news
Training at atrify - 8th training year in a row 🌟
ECR Day 2023: A look back!
On September 13 and 14, we participated in ECR Day 2023 at Kap Europa in Frankfurt - THE industry event for retail and brands. At atrify, we can look back euphorically on this successful event. Now we would like to share the most important experiences and...
The 4 most frequently asked questions about the GDSN
It's spring again - it's spring and product development is proudly presenting the new products and already during the presentation you ask yourself, what information has to be entered into the GDSN? Are these details actually mandatory? Are the new...
Are you ready for the digital transformation?
Complete and consistent product information is essential for strategic and effective brand management and successful content marketing in omni-channel. This requires optimal orchestration of data, systems and processes. But what about...
A day full of insights and inspiration at the Data Management 2023 Practice Day
On June 15, 2023, the GS1 Knowledge Center hosted this year's Data Management Practice Day. The event promised a day full of insights into the world of data management and the opportunity to learn proven strategies from experts* from various industries,...
Focus on the advantages of PIM solutions
Product Information Management solutions have become a popular topic in recent years as more and more companies realize the importance of a centralized platform for managing product information. That's why I took a closer look at the topic of...
1WorldSync expands global footprint with acquisition of atrify
1WorldSync®, the leading provider of omnichannel product content solutions, announced on May 9, 2023 the the acquisition of atrify GmbH, a wholly owned subsidiary of GS1 Germany and Europe’s largest GS1 certified product content technology and consulting partner.
atrify and EUVINO present wine labeling solution
atrify GmbH and Euvino are jointly launching the f-label solution to help producers of wine and wine-based beverages meet the new legal requirements of the Labelling Regulation in the EU. From December 2023, all...
Unlock 2023: An exciting journey through the world of PIM and PXM
On March 8 and 9, the Maison de la Mutualité in Paris hosted the Unlock Conference 2023, organized by Akeneo, the Product Experience Company. As a Solution Partner of Akeneo, we enthusiastically participated in the event and the Partner Summit....
MENA ICT 2022: Exceptional. Innovative. Futuristic.
Dream or reality?can product information exchange take off in the Middle East and beyond? That is exactly our goal at atrify! When something is impossible, we are the ones who tackle it. That's why we packed our bags and set off together with...
What does Customer Service actually do?
At the heart of atrify are our customers* - and hardly anyone interacts with them more on a daily basis than our Customer Service team. A few months ago, we thought about giving all atrifyers the opportunity to spend a day with our customers themselves.
atrify is Akeneo Solution Partner
Since September 2022, atrify has been a solution partner of Akeneo. Akeneo is a technology company that started in 2013 as a pure open source Product Information Management (PIM) and in just a few years has become one of the world's most innovative software...
Acting together. Helping together.
Social commitment is very important to us at atrify. Therefore, we have already launched several social projects in the past. It is our concern to cover as broad a spectrum as possible in order to support different areas of...
Training at atrify - what does it look like?
On September 1, 2022, five new colleagues started their training at atrify. We have already briefly announced our new trainees on our LinkedIn page - today we would also like to introduce them to you personally. In addition, we are interested in...
The 1×1 of atrify releases
A good understanding of atrify releases brings you real benefits: It ensures a better relationship with your retail customers*, as new requirements are continuously taken into account, and in addition, your consumers* also benefit from an increasing level of maturity...
novomind - now officially atrify certified
Our partner novomind has successfully passed the atrify certification. The interface of the novomind iPIM software systems was certified, which allows users to obtain and process their data in the highest quality from the atrify data pool. atrify...
How to optimize your data collection with Excel
Are you aware that the combination of data collection via Excel and our web application atrify publishing can be a real turbo for successful data exchange? And are you now wondering how you can whip your data into shape with the help of Excel...
Plytix and atrify seal PIM partnership!
Plytix Product Information Management (PIM) and atrify, one of the largest data pool operators in the world and a wholly owned GS1 subsidiary, are forming a strategic partnership to enable efficient and standardized data exchange.
ECR Day 2022: Well prepared for the re-start!
On September 28 and 29, ECR Day - the leading congress for the German consumer goods industry - will take place at the Congress Center Düsseldorf. Live. Interactive. From person to person. In 2022, the focus of the industry meeting will be on the topic "Future of Retail and...
Onboarding at atrify: This is what your start with us looks like!
What are the first weeks and months like as a new atrifyer? We wanted to know more and talked to René about it.René, you've been with us since March 1. Let's start at the beginning: How was your application process at atrify? Well...
Manafeth & atrify: First data pool in the GCC countries and Middle East
atrify and Jordan-based Manafeth Consulting & Training are joining forces to create a data pool within the trading communities of Saudi Arabia, United Arab Emirates (UAE), Kuwait, Oman, Qatar, Bahrain, Iraq and Jordan,...
Language learning with atrify
Johannes learned Spanish with atrify and put his skills to the test in South America atrify, that's about 160 colleagues from over 25 countries. And that's why our internal communication is not only in German, but also regularly in...
novomind - now officially atrify certified | atrify
Stibo Systems has undergone our comprehensive certification. The interface of the PIM solution from Stibo Systems to atrify was put through its paces, enabling users to obtain GDSN data of the highest quality from the atrify data pool and process it...
The image obligation comes
From February 2023, product images are a must While images were previously considered "nice-to-have", they will become a must-have from February 2023. This means that data exchange with at least one product image will be mandatory for data providers. Initially, the...
Meet atrify at the MedTech Forum
We are pleased to finally be back at the MedTech Forum and to welcome you to the largest healthcare and medical technology conference. Be there when experts talk about the latest trends and developments in healthcare and technology....
atrify and GS1 Austria strengthen their joint partnership
We are pleased to announce that the partnership between GS1 Austria and atrify will be strengthened again. Especially manufacturers of medical devices located in Austria can benefit from the close cooperation between GS1 Austria and atrify. From now on, they have the...
anybill declares war on the receipt obligation
Stamps, digitised. The fitness studio's course schedule, digitised. Even the long shopping list for the upcoming family celebration can be created via app and shared with your loved one. Retailers, too, are increasingly looking for digital solutions to...
atrify supports charitable associations!
Some time ago, we called on our colleagues internally to contact us with non-profit associations that need support. Many associations have experienced a very long lean period since the beginning of the pandemic, without regular operations and with less...
Training at atrify goes into the 6th round
For the 6th time, atrify is saying: Welcome to the new apprenticeship year! atrify has been successfully training apprentices for several years - and now even in four different apprenticeship professions. For "IT specialist for application development", ...
Language courses with atrify
Language as the basis of communication For an internationally operating company like atrify, with employees and customers all over the world, language exchange is not only the basis for success. The corporate culture that is so important to us is also...
Capture service now directly from atrify
Cologne, 01 July 2021. Today, the Competence Centre Data Quality of GS1 Germany was launched. The aim is to work together with industry and trade to address the issues surrounding data quality even more strongly and holistically. With the data quality service GS1 DQX...
New trees for the aTREEfy forest on World Environment Day
Have you already done something for the environment today? Today, on World Environment Day, we are once again supporting the Treedom project and are therefore having another 100 coffee and papaya trees planted in our aTREEfy company forest in Madagascar. With these trees we help...
@GP and atrify - a partnership that benefits us all
We are pleased to announce the new partnership between atrify and @GP. The French market will therefore benefit from the best of both solutions and expertise - atrify having built a successful connector to EUDAMED and other regulatory UDI databases in the world (for...
Best of Product Content
It's all there: happy customers, lots of sales, more growth Think small! At least when it comes to product content. Because if you want to successfully sell your products to women, men, children, dogs and cats, you have to make your product content...
Clean thing
Why high-quality product information is a hygiene factor The fact is: 78% of online shoppers take a closer look at product information when they order from an online shop for the first time. If we add one and one together, we can state: good and...
p36 is a certified atrify Partner!
We are pleased to announce that p36 has successfully completed the atrify certification process and is now a certified atrify partner. p36 thus enables trading partners, such as companies from the medical technology (MedTech) sector, to send data via...
Top or flop, buy or don't buy.
Why product data needs to move from being a basement child to a strategic success factor. One click: Abandonment of purchase. No one goes back and forth as quickly as customers do when shopping online. But why is that? Dr. Kai Hudetz from IFH Cologne has an answer to this question:...
Donation campaign: Giving old laptops a second chance
Online lessons, homework and talking to friends? Despite a lot of help, not every child today has this privilege, thanks to a lack of hardware. We have thought about how we as a company can help and have 14 used, fully functional...
Most needed, most wanted: Product Experience Management.
Bring on the top product data - towards the online buying experience. The very first transaction via an online shop? That was in 1994 on the US marketplace Netmarket. The payment was made online by credit card and the object of desire was a CD by Sting. Today, after almost 30...
The atrify team congratulates Joyce Engelhardt on successfully completing her training
Just recently, Joyce successfully completed her training as an office management assistant by passing her final exam in front of the IHK. Two and a half years ago, she started her training at atrify. We are very pleased about the fact that we...
Take off with data quality in 2021!
Often enough, data is called the new oil. Most of the time, companies are richly endowed with the raw material. However, the treasure trove of data is not being harnessed, as the topic neither receives sufficient attention within the company nor is it being applied to a necessary...
Donations for a good cause - Hits for the hospice
As in the last years, atrify has donated in 2020 for the good cause. This year the donation went again to the association "Hits fürs Hospiz", especially to support the project "Wünschemobil". Among other things, the non-profit association cares for seriously ill...
Getting ready for EUDAMED go-live in September 2021
EUDAMED, the key element of the Medical Device Directive (MDR) and In Vitro Diagnostics (IVDR) in the European Union, is expected to be released in modular form in the coming months. The so-called actor module will already be released in December 2020.
Traffic messages: We make product information even more efficient for you
Faster, further, more solution-oriented? This is exactly what we offer! Because we understand how important complete and high-quality product information is for your business. That's why we will be shifting up a gear in the future and with your help we will be able to improve...
How does my world get access to the GDSN - and how can a mapping service help?
Everything starts with a small and at first sight simple requirement. You are the producer of a product. You have invested all your knowledge and passion in your product and now you want to sell this product. You will find some dealers and...
atrify connects goes into the second round!
After the successful start of atrify connects in April, we are now going into the 2nd round! In the coming weeks we will continue with our free online sessions about article master data, product content & attributes. 3. november at 10:30 a.m. The...
atrify’s Support Team – Together, We Are Stronger.
We'll continue with our traffic updates: Today we will talk about who you can contact if you have any questions! Because we know how demanding data exchange with your customers can be. What makes our Customer Service special - and what do we have...
We are an excellent training company
For the fourth time in a row, the business magazine Capital honors "Germany's best trainers" and we are very pleased to be among the best trainers! Training has a high priority at atrify. It is important to us to provide our trainees with...
Product information - What are they anyway?
Product information: What is it anyway? We all come into contact with product information almost every day, whether we are shopping in the supermarket, cooking or shopping online on the couch in the evening. And these are just a few examples of where we...
The atrify health day
The first online atrify Health Day Actually, the annual and popular atrify Health Day should already take place in spring this year. Due to the pandemic, it was of course not possible to hold the event at our premises for known reasons - an...
Step by step and 80/20 rule: The development of our intuitive nutritional value editor
With release 20.09, we are launching our new Nutrition Editor: a simple and intuitive way to maintain and add to one's nutritional values within the product data.The development of the Nutrition Editor...
atrify remains colorful
... and we can get even more colorful! On the occasion of ColognePride in October in Cologne, we would also like to give an insight into our organisation and express our solidarity with the demonstrators of the CSD. We speak out clearly against discrimination of any kind....
TePe D-A-CH GmbH and atrify - Welcome to the pool
TePe has been on everyone's lips since 1965. And that in the truest sense of the word, because the Swedish company is all about healthy teeth. Interdental brushes, toothbrushes, dental picks and special brushes from TePe are in use in more than 60 countries
UDI deadline extension for high-risk equipment until January 1, 2021
China's National Medical Product Administration (NMPA) today announced an extension of the deadline for high-risk devices until January 1, 2021, which originally would have expired on October 1, 2020 for Class III high-risk devices. In the short term, the NMPA announced the...
Welcome to our data traffic news
Together with you we pursue the same goal: to get your products on as many shelves as possible. Like traffic regulations, standards such as the GDSN support a uniform, smooth and accident-free exchange of information. We know these standards are...
The atrify team congratulates Sara La Tragna on successfully completing her training
Only recently Sara successfully completed her training as an office management assistant by passing her final examination at the Chamber of Commerce and Industry. Three years ago she started her apprenticeship at atrify. We are very pleased about the fact that we can offer Sara...
The atrify team congratulates Philipp Freese on successfully completing his training
Only recently, Philipp successfully completed his training as a systems integration merchant by passing his final exams at the Chamber of Industry and Commerce. Three years ago he started his apprenticeship at atrify. We are very pleased about the fact that we...
Update on the new Saudi Arabian UDI regulation and UDI database - deadlines and procedures
The Saudi Food and Drug Authority (Saudi FDA) has confirmed the deadlines for meeting the requirements by risk class for registration in its new UDI Regulation and UDI database. You can read the full declaration in English here. If...
NOMOO and atrify - Ready for ice-cold data transfer
Delicious ice cream must also be possible in a different way - without milk. This was the idea of the two founders Rebecca Göckel and Jan Grabow in 2016. Now known under the brand name NOMOO, it is available in the chilled cabinets of almost all major retail partners. Their...
The atrify Team congratulates Manuel Commandeur on having successfully completed his apprenticeship
We are delighted that three of our trainees have successfully completed their training! In the following weeks we will report all three of them about their experiences in a mini-series. Only recently Manuel has completed his training as a commercial clerk...
New partnerships and future strategy in the healthcare sector
At atrify, we always strive to offer our customers the best possible solutions. Following the acquisition by 1WorldSync, we have been thinking hard about our future strategy and would now like to share some significant news with you. Our goal has been to...
EUDAMED preparation made easy: Your indispensable checklist with 10 key steps
EUDAMED is the central information system developed by the European Commission to increase transparency and improve traceability of medical devices in the European Union. As the UDI module is already in production environment...
The impact of EUDAMED registration on supply chain management in the medical device industry
The introduction of EUDAMED has a significant impact on supply chain management in the medical device industry. However, these positive effects can only be optimized if they are combined with a strategy for exchanging product information with...
Recap EUDAMED Online Session - MedTech Europe
I am honored to have been invited as a speaker for the MedTech Europe webinar on EUDAMED, which took place at the end of March. The webinar was organized specifically for the MedTech industry and had over 1,000 registered participants, a...
Did you know that on average, manufacturers are missing 60% of the data required in EUDAMED?
We tell you how you can close this gap! In many gap analyses we have conducted, we have found that medical device manufacturers are missing on average about 60% of the data required by EUDAMED. This shows how important it is to...
The revision of the EU MDR: Beauty products now fall under the Medical Devices Regulation
The EU Medical Device Regulation (EU MDR) covers certain beauty products that were previously not considered medical devices. This means that these products must now meet the same safety and efficacy standards as conventional medical devices.The...
Deadline extensions for legacy products do not affect EUDAMED deadlines
On Dec. 9, the European Commission announced the extension of the validity period of certificates for legacy products on the European market. This has caused uncertainty and perplexity among some medical device manufacturers regarding the...
Meet atrify and USDM at the MEDTECH Conference 2022 in Boston
The AdvaMed MedTech Conference is the top event for medtech professionals. World-class speakers, an informative program, networking with professionals from the medtech industry and plenty of business opportunities make the Medtech Conference an event that medtech...
Visit us at the RAPS Convergence in Phoenix
Visit atrify at our joint booth TT-14 with USDM Life Sciences at RAPS Convergence, September 11-13, 2022 in Phoenix, Arizona. RAPS Convergence is the world's largest annual gathering of professionals and innovators in the field of...
Visit us at the AHRMM22 in Anaheim
Visit atrify at booth 529 at the AHRMM22 Conference & Exhibition, August 7-10, 2022 at the Anaheim Convention Center in Anaheim, California.The AHRMM22 Conference & Exhibition is the premier showcase for the latest products, solutions,...
Lionel Tussau reelected Member of the GS1 Healthcare Leadership Team
I'm very happy to confirm that for the second year, our representative Lionel Tussau has been reelected Member of the GS1 Healthcare Leadership Team, in charge of leading the strategy of GS1 worldwide for this industry. We thank the community for their vote of...
EUDAMED - mandatory use in Finland!
We'd like to remind you that all medical devices sold in Finland must be registered in EUDAMED (UDI module) - whether new or "legacy devices" - since 2021; and the same will apply to IVD legacy devices by August 2022 already! With atrify, you can easily comply with...
GS1 Norway recommends atrify's UDI Solution for EUDAMED registration
Even if the mandatory registration of medical devices in EUDAMED still seems a long way off until 2023, many experts recommend already dealing with the complex requirements now and start testing and registering in time. GS1 Norway officially recommends using the...
"UDI and EUDAMED" session with Lionel Tussau at the Italian Regulatory Affairs Days Round Table
Lionel Tussau, the head of atrify Healthcare Market Unit, will participate in the round table organized by the Italian Trade Association Confindustria Dispositivi Medici during the Regulatory Affairs Days on 27th May (digital event), to share the expertise of the...
Meet atrify at the MedTech Forum
We are pleased to finally be back at the MedTech Forum and to welcome you to the largest healthcare and medical technology conference. Be there when experts talk about the latest trends and developments in healthcare and technology....
Why companies who went through atrify's Attribute Analysis have been successful
For the last few months, you have been the "happy" project leader for Eudamed! The one who inherited this project that nobody wanted... This topic has already put you in a cold sweat, just trying to get your famous SRN... Now that the UDI module is live (since last...
The time for Scan 4 Safety is NOW!
After years of zoom meetings, it was great to see so many of you in real life. This year's conference was heavily focused on the Scan 4 Safety project, for which atrify provides the GDSN data pool. Healthcare in the UK is starting to move forward beyond COVID, but...
Release 22.04: Final Release Notes atrify publishing & approval
In this release, the focus is on: Verified Recipients, CIN Process optimisation Change Application Type of change Added value for you Introduction of a new publication target publishing and datapool Improvement in the maintenance of publication targets More transparency about...
atrify and GS1 Austria strengthen their joint partnership
We are pleased to announce that the partnership between GS1 Austria and atrify will be strengthened again. Especially manufacturers of medical devices located in Austria can benefit from the close cooperation between GS1 Austria and atrify. From now on, they have the...
Meet us at the GS1 UK Healthcare Conference in London
Finally, the live events are back! atrify will have a stand at the GS1 UK Healthcare Conference in London on 17 and 18 March 2022. This year, the focus will be on how GS1 standards are being used to improve patient safety and...
New UK consultation by the MHRA on the regulation of clinical trials
After its consultation on the UDI regulation in the UK, the MHRA is now holding another consultation on the Clinical trial regulation to improve processes and simplify the activity of clinical trial sponsors. Its focus is particularly on "clinical trials and the...
medical.device.forum of TÜV Süd: atrify gives tips on UDI registration in EUDAMED
Hosting a forum during a pandemic can be quite a challenge, especially when the participants are from the health sector. TÜV Süd, a notified body, has risen to the challenge and made the best of the...
EUDAMED - From expertise to solution
The EUDAMED UDI module for the registration of medical devices has been in operation since 4 October 2021 - voluntary from the point of view of the European regulation, mandatory or almost mandatory according to various European member states. Reminder: 5 years...
atrify supports charitable associations!
Some time ago, we called on our colleagues internally to contact us with non-profit associations that need support. Many associations have experienced a very long lean period since the beginning of the pandemic, without regular operations and with less...
UK UDI Regulation - Consultation from the MHRA
The UK regulator MHRA has launched an official Consultation on their future UDI regulation due to close on 26th November 2021. The regulation is expected to enter into force in July 2023. The consultation itself is very thorough, looking at a number of options for...
China UDI Database (CUDID) - Updated deadline for Class III registration
The Chinese authorities have confirmed that all remaining Class III medical devices must be registered in the CUDID (NMPA UDI database) by 1 June 2022 - 8 months from now. atrify has been associated with the CUDID for years. The...
EUDAMED UDI module live!
Following the release of the Actor module, which has been operational since December 2020, the UDI and CERTIFICATE modules went live in EUDAMED on Monday 4 October. This is another important step towards the implementation of EUDAMED and the MDR/IVDR....
@GP and atrify - Joint Online Session for the French Community - 23.09.2021
As an ongoing effort to provide information on regulations, atrify hosts online sessions in multiple languages on EUDAMED, UDI regulations, and the exchange of product content between trading partners. @GP and atrify are hosting a joint online session "UDI, EUDAMED,...
Training at atrify goes into the 6th round
For the 6th time, atrify is saying: Welcome to the new apprenticeship year! atrify has been successfully training apprentices for several years - and now even in four different apprenticeship professions. For "IT specialist for application development", ...
Language courses with atrify
Language as the basis of communication For an internationally operating company like atrify, with employees and customers all over the world, language exchange is not only the basis for success. The corporate culture that is so important to us is also...
The atrify team congratulates Timo Meßner on successfully completing his training
Just recently, Timo successfully completed his training as an IT specialist for system integration with us by passing his final exam at the IHK. Timo started his training at atrify just under three years ago and we are very pleased...
My internship at atrify
My school internship was supposed to have taken place last year, but was postponed to this year due to the Corona pandemic, so I started my three-week internship in the marketing department at atrify in June 2021. Since my father works at...
Capture service now directly from atrify
Cologne, 01 July 2021. Today, the Competence Centre Data Quality of GS1 Germany was launched. The aim is to work together with industry and trade to address the issues surrounding data quality even more strongly and holistically. With the data quality service GS1 DQX...
Summer break - Welcome to the last UDI playground of EUDAMED
Healthcare manufacturers will have 1 more chance to participate in the EUDAMED playground for UDI & Device registration module which will be opened by the European Commission at the end of July and run during the month of August, enabling direct interaction with...
@GP and atrify - Joint Online Session for the French Community
As an ongoing effort to provide information on regulations, atrify hosts online sessions in multiple languages on EUDAMED, UDI regulations, and the exchange of product content between trading partners. @GP and atrify are hosting a joint online session "UDI, EUDAMED,...
The European MDR is live!
On 26th May 2021, the EU Medical Devices Regulation (MDR) finally came into full application, superseding the former medical device directives from the 1990's. EUDAMED has been live since December 2020 (Actor Registration module) and its 2 next modules (UDI and...
Time, costs, nerves. All saved!
Product Experience Management with the help of external service providers Sorry, no comment! This might still happen after debates in the Bundestag or disastrous football matches. But online, the silence has long since ended. Here we can talk about...
Updates in the Healthcare Industry
There are a number of regulatory changes happening in healthcare right now that companies need to keep a close eye on - and we're here to help. Firstly, changes to the EUDAMED deadlines for the UDI and device registration module will make it easier for the industry to...
@GP and atrify - a partnership that benefits us all
We are pleased to announce the new partnership between atrify and @GP. The French market will therefore benefit from the best of both solutions and expertise - atrify having built a successful connector to EUDAMED and other regulatory UDI databases in the world (for...
Best of Product Content
It's all there: happy customers, lots of sales, more growth Think small! At least when it comes to product content. Because if you want to successfully sell your products to women, men, children, dogs and cats, you have to make your product content...
Clean thing
Why high-quality product information is a hygiene factor The fact is: 78% of online shoppers take a closer look at product information when they order from an online shop for the first time. If we add one and one together, we can state: good and...
Top or flop, buy or don't buy.
Why product data needs to move from being a basement child to a strategic success factor. One click: Abandonment of purchase. No one goes back and forth as quickly as customers do when shopping online. But why is that? Dr. Kai Hudetz from IFH Cologne has an answer to this question:...
Donation campaign: Giving old laptops a second chance
Online lessons, homework and talking to friends? Despite a lot of help, not every child today has this privilege, thanks to a lack of hardware. We have thought about how we as a company can help and have 14 used, fully functional...
EUDAMED - The countdown is on! Making the leap from GUDID to EUDAMED
EUDAMED is getting closer every day and testing is about to restart for the last UDI registration playground before going live in a couple of months. Time is getting short for companies to get their processes and UDI data in order. How to be compliant with the MDR...
Most needed, most wanted: Product Experience Management.
Bring on the top product data - towards the online buying experience. The very first transaction via an online shop? That was in 1994 on the US marketplace Netmarket. The payment was made online by credit card and the object of desire was a CD by Sting. Today, after almost 30...
The atrify team congratulates Joyce Engelhardt on successfully completing her training
Just recently, Joyce successfully completed her training as an office management assistant by passing her final exam in front of the IHK. Two and a half years ago, she started her training at atrify. We are very pleased about the fact that we...
Take off with data quality in 2021!
Often enough, data is called the new oil. Most of the time, companies are richly endowed with the raw material. However, the treasure trove of data is not being harnessed, as the topic neither receives sufficient attention within the company nor is it being applied to a necessary...
Healthcare manufacturers are now able to reach French GPO Resah via GDSN
As a leading provider of product content solutions, we are pleased to announce that healthcare manufacturers are now able to send their data to Resah via the Global Data Synchronization Network (GDSN) for consolidated and reliable...
SRC System Integrators & atrify - Creating great value for customers by working together
The partnership between SRC System Integrators and atrify can especially benefit customers located in the Netherlands! SRC System Integrators (SRC) offers solutions that ensure the accuracy of product information and facilitate the exchange of...
Getting ready for EUDAMED go-live in September 2021
EUDAMED, the key element of the Medical Device Directive (MDR) and In Vitro Diagnostics (IVDR) in the European Union, is expected to be released in modular form in the coming months. The so-called actor module will already be released in December 2020.
EUDAMED Actor Registration Live
atrify welcomes the production go-live of the EUDAMED Actor Registration module on 1st December 2020 This is a major milestone towards enabling manufacturers to be MDR and EUDAMED compliant, ahead of the production opening of UDI Device registration and Certificates...
The modular platform for the beverage industry - Euvino and atrify
Partnership with predicate: Together we make ourselves strong for our customers for more efficiency and more reach. We will drink a toast to this: Euvino, the modular platform for the beverage industry, and atrify, the leading product content platform for the beverage industry...
How does my world get access to the GDSN - and how can a mapping service help?
Everything starts with a small and at first sight simple requirement. You are the producer of a product. You have invested all your knowledge and passion in your product and now you want to sell this product. You will find some dealers and...
atrify connects goes into the second round!
After the successful start of atrify connects in April, we are now going into the 2nd round! In the coming weeks we will continue with our free online sessions about article master data, product content & attributes. 3. november at 10:30 a.m. The...
atrify’s Support Team – Together, We Are Stronger.
We'll continue with our traffic updates: Today we will talk about who you can contact if you have any questions! Because we know how demanding data exchange with your customers can be. What makes our Customer Service special - and what do we have...
We are an excellent training company
For the fourth time in a row, the business magazine Capital honors "Germany's best trainers" and we are very pleased to be among the best trainers! Training has a high priority at atrify. It is important to us to provide our trainees with...
Product information - What are they anyway?
Product information: What is it anyway? We all come into contact with product information almost every day, whether we are shopping in the supermarket, cooking or shopping online on the couch in the evening. And these are just a few examples of where we...
atrify's Collaboration with GridAgent Proving Successful
We are pleased to announce that ever since GridAgent joined atrify's Partner Program in 2019, our combined strengths have been enabling a growing number of Japanese customers to comply accurately and efficiently with global requirements in the healthcare industry....
The atrify health day
The first online atrify Health Day Actually, the annual and popular atrify Health Day should already take place in spring this year. Due to the pandemic, it was of course not possible to hold the event at our premises for known reasons - an...
Step by step and 80/20 rule: The development of our intuitive nutritional value editor
With release 20.09, we are launching our new Nutrition Editor: a simple and intuitive way to maintain and add to one's nutritional values within the product data.The development of the Nutrition Editor...
atrify remains colorful
... and we can get even more colorful! On the occasion of ColognePride in October in Cologne, we would also like to give an insight into our organisation and express our solidarity with the demonstrators of the CSD. We speak out clearly against discrimination of any kind....
TePe D-A-CH GmbH and atrify - Welcome to the pool
TePe has been on everyone's lips since 1965. And that in the truest sense of the word, because the Swedish company is all about healthy teeth. Interdental brushes, toothbrushes, dental picks and special brushes from TePe are in use in more than 60 countries
UDI deadline extension for high-risk equipment until January 1, 2021
China's National Medical Product Administration (NMPA) today announced an extension of the deadline for high-risk devices until January 1, 2021, which originally would have expired on October 1, 2020 for Class III high-risk devices. In the short term, the NMPA announced the...
Welcome to our data traffic news
Together with you we pursue the same goal: to get your products on as many shelves as possible. Like traffic regulations, standards such as the GDSN support a uniform, smooth and accident-free exchange of information. We know these standards are...
The atrify team congratulates Sara La Tragna on successfully completing her training
Only recently Sara successfully completed her training as an office management assistant by passing her final examination at the Chamber of Commerce and Industry. Three years ago she started her apprenticeship at atrify. We are very pleased about the fact that we can offer Sara...
The atrify team congratulates Philipp Freese on successfully completing his training
Only recently, Philipp successfully completed his training as a systems integration merchant by passing his final exams at the Chamber of Industry and Commerce. Three years ago he started his apprenticeship at atrify. We are very pleased about the fact that we...
Update on the new Saudi Arabian UDI regulation and UDI database - deadlines and procedures
The Saudi Food and Drug Authority (Saudi FDA) has confirmed the deadlines for meeting the requirements by risk class for registration in its new UDI Regulation and UDI database. You can read the full declaration in English here. If...
NOMOO and atrify - Ready for ice-cold data transfer
Delicious ice cream must also be possible in a different way - without milk. This was the idea of the two founders Rebecca Göckel and Jan Grabow in 2016. Now known under the brand name NOMOO, it is available in the chilled cabinets of almost all major retail partners. Their...
The atrify Team congratulates Manuel Commandeur on having successfully completed his apprenticeship
We are delighted that three of our trainees have successfully completed their training! In the following weeks we will report all three of them about their experiences in a mini-series. Only recently Manuel has completed his training as a commercial clerk...
Healthcare User Group Meetings
At atrify we value the close exchange with our customers. For this reason, regular user group meetings are held for clients from the healthcare industry. The work of these groups is incorporated into our roadmap and ensures that the solutions we develop are implemented.
Getting compliant with China's UDI deadline of Oct 1, 2020 for high risk devices with atrify
Note of 30 September 2020: Extension of China's UDI deadline for Class III high-risk devices until 1 January 2021 Original contribution: Compliance with China's UDI deadline of 1 October 2020 for high-risk devices with atrify. The UDI registration requirements...
China Customs GDSN Initiative! Meet all requirements with atrify’s GDSN data pool!
Data rules the world. The steady increase in the import and export of goods to China prompted the authorities to look for a solution to make data exchange more efficient. The Global Data Synchronization Network (GDSN) has been recognized as the leading...
atrify goes green - JobRad for everyone!
There it is - the JobRad for atrify The birds are chirping, the sun is shining and it is getting longer and brighter again - the bike spring is here! And also the long awaited JobRad for atrify. This was the official information from atrify to announce the participation in the...
GS1 Healthcare GTIN Allocation Rules® updated and published
In June 2020, the GS1 Healthcare Global Trade Item Numer (GTIN) Allocation Rules were updated to improve their application rules, supply chain and point of care applications. Click here for the English Healthcare GTIN Allocation Rules...
Correctly addressing GDSN XML messages in the GDSN
Based on the last blog "GDSN Machine-To-Machine (M2M) - the supreme discipline of electronic data exchange - an overview", this blog is about addressing XML messages correctly. In this blog I explain the most common confusions with regards to addressing messages, when...
Responding to the COVID-19 Pandemic: the Diagnostic Industry Angle
As part of its involvement against the Covid-19, atrify is participating in a number of initiatives.
atrify is now an official member of the GS1 Healthcare US Initiative
We are proud to announce that atrify is now an official GS1 Healthcare US Initiative Member! With this membership, atrify and its customers will now participate directly in shaping the future of the healthcare industry for the best outcomes for patients and the healthcare industry as a whole in the US and beyond.
From product to shelf with atrify
We support food start-ups to get on the shelf of the retail trade. Why we can do this so well we explain in our video. Free Webinar How you can best get a place on the supermarket shelf and be supported in doing so, you can find out in...
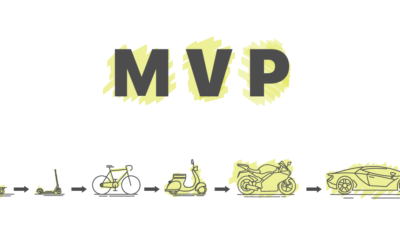
Early Feedback - The MVP Thought
There is hardly a term for which, when I ask different people about its meaning, I get so many different answers as with MVP. For some, an MVP is already a fully developed piece of...
The atrify team congratulates Eleni Stavridou on successfully completing her training
This year Eleni successfully completed her training as an office management assistant by passing her final examination at the Chamber of Commerce and Industry. Two and a half years ago she started her apprenticeship at atrify. We are very pleased about the fact that...