The European Commission issued two new regulations in 2017: firstly, the Medical Device Regulation 2017/745/EU, or MDR for short. And secondly, the In Vitro Diagnostic Medical Devices Regulation 2017/746/EU, known as IVDR. A lot of progress has been made in both areas over the last 20 years. It is therefore high time to manifest the goals that have been achieved, adapt key elements and make the content more transparent for everyone - from manufacturers to patients.
In future, the MDR will replace the existing Medical Device Directive(93/42/EEC - MDD) and the Active Implantable Medical Devices Directive(90/385/EEC - AIMDD). Important to know: In contrast to directives, European regulations do not have to be transferred to the respective national law. This means that the regulations are implemented in the same way for all EU member states.
The MDR was published in May 2017, marking the start of a three-year transition phase during which the AIMDD and MDD will be replaced by the new Medical Device Regulation. The MDR will come into force in May 2020, from which time stakeholders will be able to voluntarily comply with the regulation. The data must then be uploaded in accordance with the new MDR by November 2021. While the MDR will be valid from May 2020, the IVDR will not come into force until 2022. It is now important for all stakeholders involved in medical devices to comply with both regulations.
What will change for you?
When a directive becomes a regulation, we speak of new requirements rather than amendments. This is because the direction already taken remains the same. In the case of the new Medical Devices Regulation, this means that the previous requirements all remain in place, but some new features have been added. Here is an excerpt of the most important points:
- Introduction of a new, unique identification system (UDI - Unique Device Identification)
- Appointment of a person responsible for compliance with the regulations.
- Appointment of an authorized representative if the manufacturer is based outside the EU (Authorized Representative)
Where exactly does EUDAMED come in?
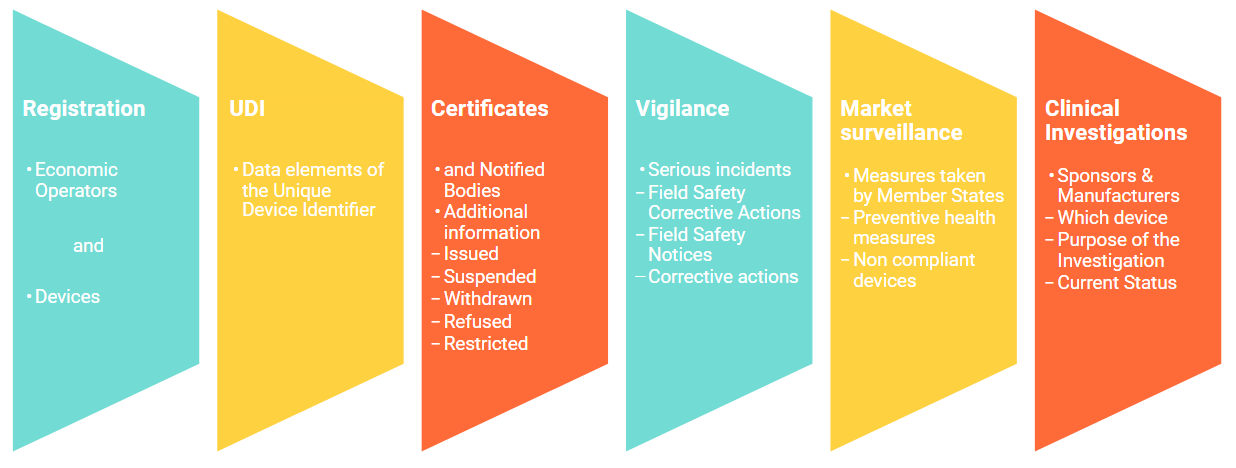
One thing is certain: The identification of approved devices is one of the main elements arising from the new regulations. This naturally entails large volumes of data. A European database for medical devices, "EUDAMED", has been developed in order to manage the quantity and quality of the collected data securely and optimally. This European Database on Medical Devices is an Internet portal that manufacturers use independently to enter and maintain their medical devices. This database is divided into the following modules:
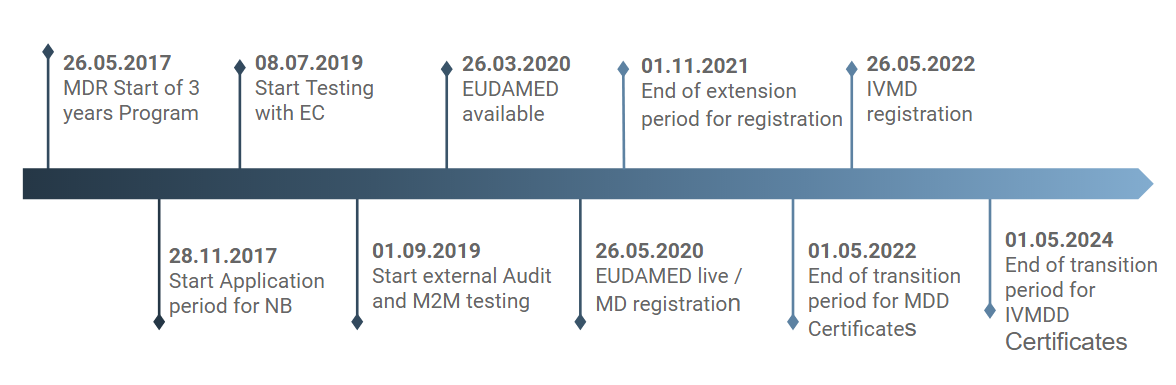
Which important dates should you have noted down?
To give you a precise overview of the next milestones, you will find the most important dates and facts here (as at August 2019).
Where are we now?
A lot has happened since May 2017. We can therefore already make concrete statements on some points:
- An extension of 18 months was granted for product registration. However, there are some exceptions here, which mainly relate to clinical monitoring and patient safety (until 11/2021).
- EUDAMED will not be fully functional upon activation. Using a roadmap, those involved can precisely track the timing for implementation; 4 releases are planned here:
- High (1): first release in March 2020
- High (2): second release in November 2020
- Medium (3): third release in May 2021
- Medium (4): fourth release in May 2022
- Low (5): later release (not for audit)
And atrify?
- atrify actively participates in the exchange between manufacturers, the European Commission and MedTech Europe. MedTech represents the medical technology industry.
- atrify is working on the AS4 connection and is currently preparing the XML file. This file will serve as the basis for communication with EUDAMED.
It is very exciting for us to see how the implementation is progressing. We know how crucial implementation is for everyone involved and look forward to realizing the next steps. Of course, compliance with the MDR presents manufacturers with a new challenge. That's why we are doing everything we can to support manufacturers with our solutions.
What happens next?
As you know, we are only at the beginning of the EUDAMED story. That is why we will keep you up to date in the future and inform you regularly about changes, news and improvements.
Find out more about our EUDAMED solution.




