
Global Medical Device Registration Made Easy:
A Guide for Manufactures
Download atrify's free ebook to receive guidance on how to register your medical devices worldwide.
A comprehensive approach to achieving global UDI compliance
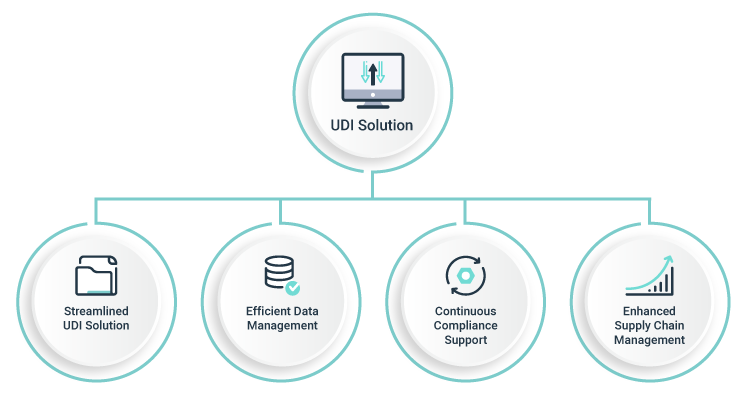
Manual approaches to UDI compliance can be time-consuming and error-prone, leading to compliance issues and potentially costly consequences. Acomprehensive UDI solution can provide medical device manufacturers with the tools and resources they need to achieve and maintain global UDI compliance.
The benefits of a comprehensive UDI solution are numerous, including
- improved compliance
- enhanced data management
- better supply chain management and finally a safe and
- valid registration of all medical devices

Global Medical Device Registration
- Let us show you how!
This eBook covers the following topics:
- UDI System Implementation and Global Requirements
- UDI Registration: Why It Matters
- A Comprehensive Approach to Achieving Global UDI Compliance
- Checklist for Global UDI Registration Strategy
- Streamlining UDI Data Management with the atrify UDI Solution
Download free eBook + checklist now
Get an in-depth guide to achieving global UDI compliance and learn how to streamline worldwide UDI registrations.
