
Global Medical Device Registration Made Easy:
A Guide for Manufactures
Download atrify’s free ebook to receive guidance on how to register your medical devices worldwide.
A comprehensive approach to achieving global UDI Compliance
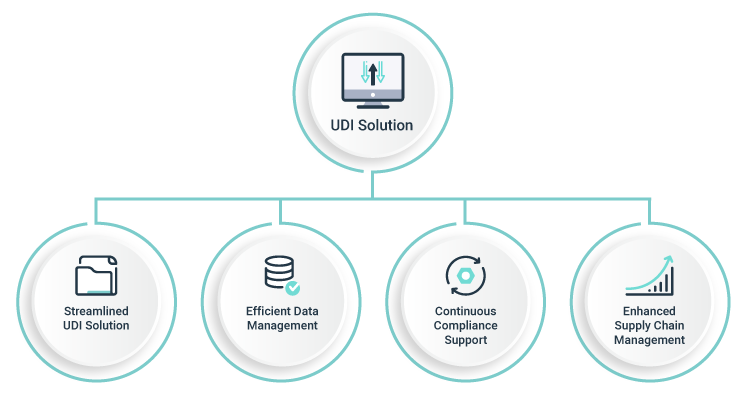
Manual approaches to UDI compliance can be time-consuming and error-prone, leading to compliance issues and potentially costly consequences. A comprehensive UDI solution can provide medical device manufacturers with the tools and resources they need to achieve and maintain global UDI compliance.
The benefits of a comprehensive UDI solution are numerous, including
- improved compliance
- enhanced data management
- better supply chain management and finally a safe and
- valid registration of all medical devices

Global Medical Device Registration
– Let us show you how!
This eBook covers the following topics:
- UDI System Implementation and Global Requirements
- UDI Registration: Why It Matters
- A Comprehensive Approach to Achieving Global UDI Compliance
- Checklist for Global UDI Registration Strategy
- Streamlining UDI Data Management with the atrify UDI Solution
Download free eBook + checklist now
Get an in-depth guide to achieving global UDI compliance and learn how to streamline worldwide UDI registrations.
